During cellular respiration sugar is broken down to carbon dioxide and water and ATP is made. View solution Combustion is a _____ reaction accompanied by heat and light.
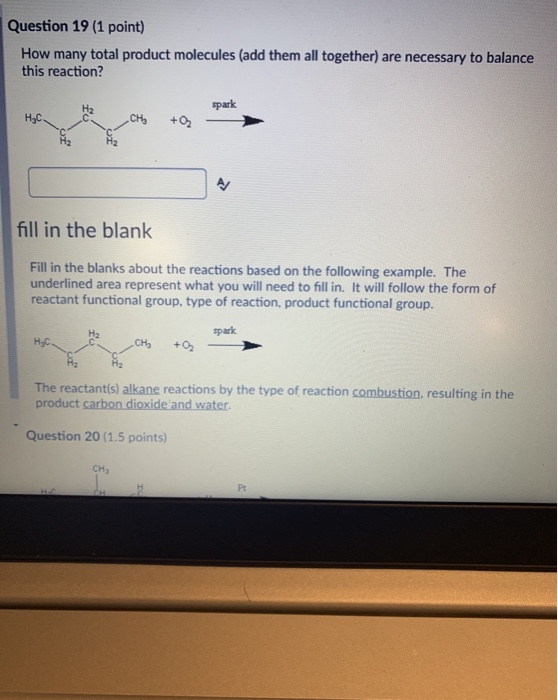
Solved Question 19 1 Point How Many Total Product Chegg Com
If two reactant particles collide but a reaction does not occur this is called a n _____.

. It is not necessary to sketch out the molecules - just write the chemical formulas. Write your observations for the following chemical reactions and name the products formed. The following is not a combustion reacti.
View solution What must be present for a combustion reaction to occur. In a combustion reaction energy is released along with the formation of CO 2. Combustion is only possible if the substance is combustible ie.
The cleaner it burns the more complete the combustion. Use the potential energy diagram to answer the question. Conditions necessary for Combustion.
The substances which can burn. Fuel oxygen and heat. D It is the oxidation reaction where.
In a particular reaction vessel 45 mol of H2 are reacted with 45 mol of N2. Answer Expert Verifiedquestionquestion mark. Oxygen is the necessary constituent for any combustion reaction as it is a supporter of combustion and cutting the supply of oxygen will stop the combustion immediately.
When water is added to quick lime. Oxygen is necessary for combustion. A all carbon always becomes CO2 B high octane fuels release more energy C all hydrogen always becomes water D all carbon always becomes CO.
It is commonly called burning. The reactant necessary for cellular respiration to occur is oxygen. The following conditions are necessary for combustion to take place-Presence of a combustible substance.
Atoms Chemical Kinetics Moving Charges and Magnetism Microbes in Human. B It is the combustion of ethanol with the formation of carbon dioxide and water. C It is the complete combustion of carbon monoxide to form carbon dioxide.
The energy necessary to align the reactant molecules and cause them to collide with enough energy to form products is. The other reactant usually contains carbon and hydrogen and sometimes oxygen too but only if the non-oxygen reactant is a. Octane oxygen 113-trimethyl cyclohexane oxygen 4-ethyl-25-dimethylheptane oxygen chemical equations as representing a Type - Classify each of the following balanced chemical equations ynthesis.
- Give reagents necessary to synthesize the following molecules Question Also can you provide an explanation along with the drawing as to how you come up with the answer please. During combustion substance reacts with an oxidising agent such as. A Classify each substance as either a reactant or product in the chemical reaction.
Oxygen Combustion requires oxygen. Cellular respiration in a sense is breaking down sugar in the presence of oxygen. Use the graph to answer the question When a catalyst is used the activation energy is.
What is essential for a combustion reaction to begin. A It is combustion of Methane where carbon dioxide and water are formed. Oscillations Redox Reactions Limits and Derivatives Motion in a Plane Mechanical Properties of Fluids.
When all substances in a compound combine with oxygen and produces. The reactant that produces the least amount of possible product is the limiting reactant. A type of decomposition reaction and are also oxidation reduction reactions.
In a combustion reaction ethanoic acid CH3COOHCH3COOH is burned in the presence of oxygen O2O2 producing carbon dioxide CO2CO2 and water H2OH2O. ATP is then used for cellular work. Hydrogen and nitrogen react to form ammonia according to the balanced equation 3H2 g N2 g 2NH3 g.
This problem has been solved. Write the balanced equations for the following combustion reactions. Refers to the efficiency of the combustion.
B Starting with the unbalanced equation for the combustion of ethanoic acid. In the following reaction oxygen is the excess reactantSiCl4 O2 SiO2 Cl2The table shows an experimental record for the above reaction. Examples-wood paper kerosene oil straw match stick etc.
Let have a look at the options. Experimental Record Trial Starting Amount of SiCl4 Starting Amount of O2 Actual Yield of SiO2 1 150 g 200 g 492 g 2 75 g 50 g 252 g a. Which of the following is true in combustion reactions.
Calculate the percentage yield for SiO2 for.

Which Reactant Is Necessary For A Combustion Reaction Brainly Com
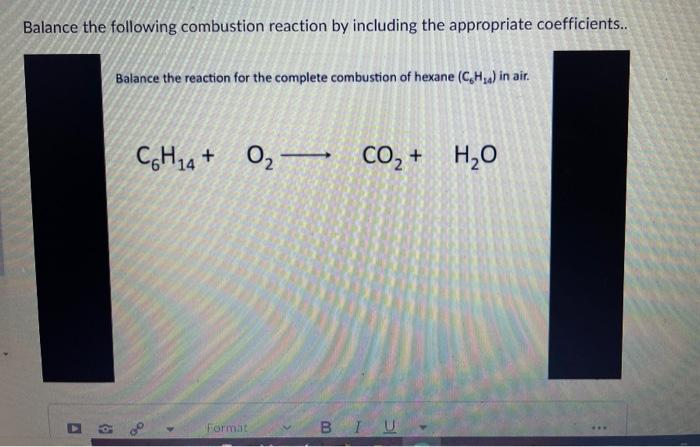
Solved Balance The Following Combustion Reaction By Chegg Com
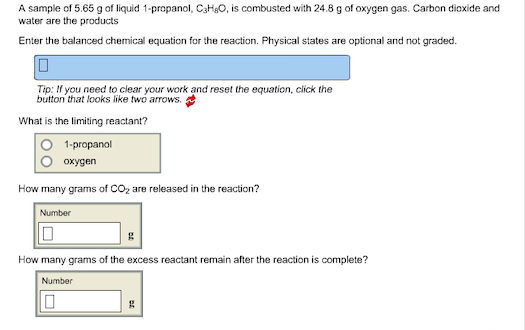
Solved Balance The Following Combustion Reaction In Order To Chegg Com
0 Comments